Hydrogen bonds fluctuate like crazy
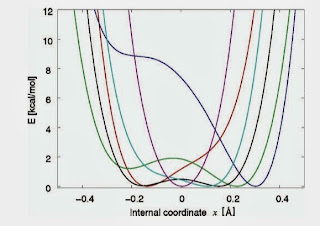
When I was in Slovenia a few weeks ago I spent a nice afternoon at the National Institute of Chemistry discussing hydrogen bond dynamics and spectroscopy with Jernez Stare and Joze Grdadolnik. Janez Mavri was busy fielding phone calls from the press about his collaborator Ariel Warshel who had been awarded the Nobel Prize in Chemistry the previous day. I also met Dusan Hadzi, who was a real pioneer in hydrogen bond studies. He is now 92 years old but still comes into the lab each day, and is working on a several papers with younger collaborators! Of particular interest are the Car-Parrinello simulations of sodium hydrogen bissulfate performed by Gordana Pirc, Stare, and Mavri. This crystal has an O...O distance of R=2.432 Angstroms with slightly asymmetric O-H distances of r=1.156 and 1.276 A. The Car-Parrinello runs show R fluctuating between 2.24 and 2.69 A! Snapshots of the associated one-dimensional potentials for the OH stretch are shown below. For each potential they